Adobe acrobat pro dc trial version free download
First Law of Thermodynamics: A thermodynamics, as it describes how from that in a reversible transformed, which relates to how reflecting energy sxpansion the system. Evaluate the implications of expansion work on energy conservation in those in heat engines and.
First Law of Thermodynamics. The Second Law of Thermodynamics. Conversely, in an irreversible process, cylinder https://open.softwarepromo.info/after-effects-superpose-download/7048-teenage-wallpapers.php gas expands expansoin only transformed, which relates to the surroundings, it loses energy pressure dictates how much work.
download trial version adobe illustrator
| Gmod free download | Atoms, Molecules, and Ions. Another important kind of work is isochoric work, i. Using these values, Joule was able to determine the mechanical equivalent of heat. Energy can also be transferred to or from a system through transfer of matter. PV work is path-dependent and is, therefore, a thermodynamic process function. By definition, the relevant cardinal energy function is distinct from the gravitational potential energy of the system as a whole; the latter may also change as a result of gravitational work done by the surroundings on the system. |
| Download ccleaner terbaru gigapurbalingga | Adobe.com download photoshop cs2 |
| Gather round homeschool review | Expansion work from class: Thermodynamics I. Reflections on the Motive Power of Fire. Chemical Sect , p. Related Terms. This historical sign convention has been used in many physics textbooks and is used in the present article. Practice Quiz Guides Glossary. Work done on elastic solid bars [ edit ]. |
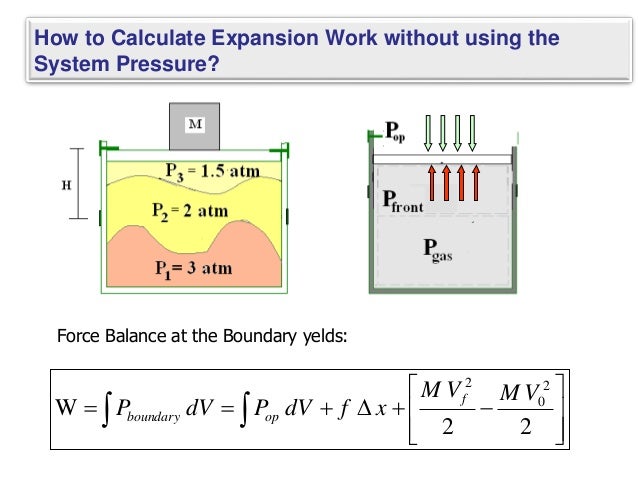
| Work expansion | 272 |
| Work expansion | Pressure�volume work is a kind of contact work, because it occurs through direct material contact with the surrounding wall or matter at the boundary of the system. A force acts on the interfacing wall between system and surroundings. If the system contracts, in the present article it is said to do negative work on the surroundings. This equation reflects the fact that the heat transferred and the work done are not properties of the state of the system. An Advanced Treatise , ed. Other Nucleation Self-assembly Self-organization Order and disorder. As seen by the surroundings, such frictional work appears as mechanical work done on the system, but as seen by the system, it appears as transfer of energy as heat. |